
In recent years, medications like Wegovy (Semaglutide) and Mounjaro (Tirzepatide) have taken the world by storm, revolutionising the management of Type 2 diabetes and obesity. Their profound impact on blood sugar control and weight loss has generated headlines and offered new hope to millions. But what if this was just the beginning? What if the next generation of metabolic medicine could deliver even more powerful results?
Enter Retatrutide, an investigational medicine that is rapidly becoming one of the most talked-about drugs in development.
Nicknamed the "Triple G" drug, Retatrutide is currently undergoing extensive Phase 3 clinical trials and is not yet available to the public.
Its unique power lies in its ability to target not one, not two, but three key metabolic hormone receptors, a triple-action mechanism that promises to push the boundaries of what's possible in diabetes and weight management.
This article will delve into the ground-breaking science to discover how Retatrutide could revolutionise diabetes and weight loss management with its triple-agonist action.
How Retatrutide Works: The 'Triple G' Advantage
The excitement surrounding Retatrutide stems from its sophisticated and unprecedented mechanism of action. While older drugs target a single hormone pathway, Retatrutide is a triple receptor agonist, engaging with three distinct hormones that play a crucial role in regulating our metabolism, appetite, and blood sugar. This multi-pronged attack is what sets it apart and gives it a potential clinical edge.
To understand its power, we need to break down each component of its "Triple G" action:
- GLP-1 (Glucagon-like peptide-1) Receptor Agonism: This is the same mechanism used by drugs like Ozempic and Wegovy. Activating the GLP-1 receptor tells the brain you are full, significantly reducing appetite. It also slows down the rate at which your stomach empties, prolonging the feeling of satiety after a meal. For individuals with Type 2 diabetes, it stimulates the pancreas to release insulin in response to high blood sugar levels.
- GIP (Glucose-dependent insulinotropic polypeptide) Receptor Agonism: This is the second target, which Retatrutide shares with the dual-agonist drug Mounjaro. GIP also enhances the body's insulin release in response to glucose, working synergistically with GLP-1 to improve blood sugar control. Furthermore, research suggests GIP may play a role in how the body processes and stores fat.
- Glucagon Receptor Agonism: This is Retatrutide's unique and game-changing feature. While it might seem counterintuitive to activate the receptor for glucagon, a hormone that raises blood sugar, the effect in this context is profoundly different. Activating the glucagon receptor in combination with GLP-1 and GIP appears to increase energy expenditure. In simple terms, it helps the body burn more calories and fat, even at rest. This action specifically targets the liver, reducing fat accumulation and improving overall metabolic health.
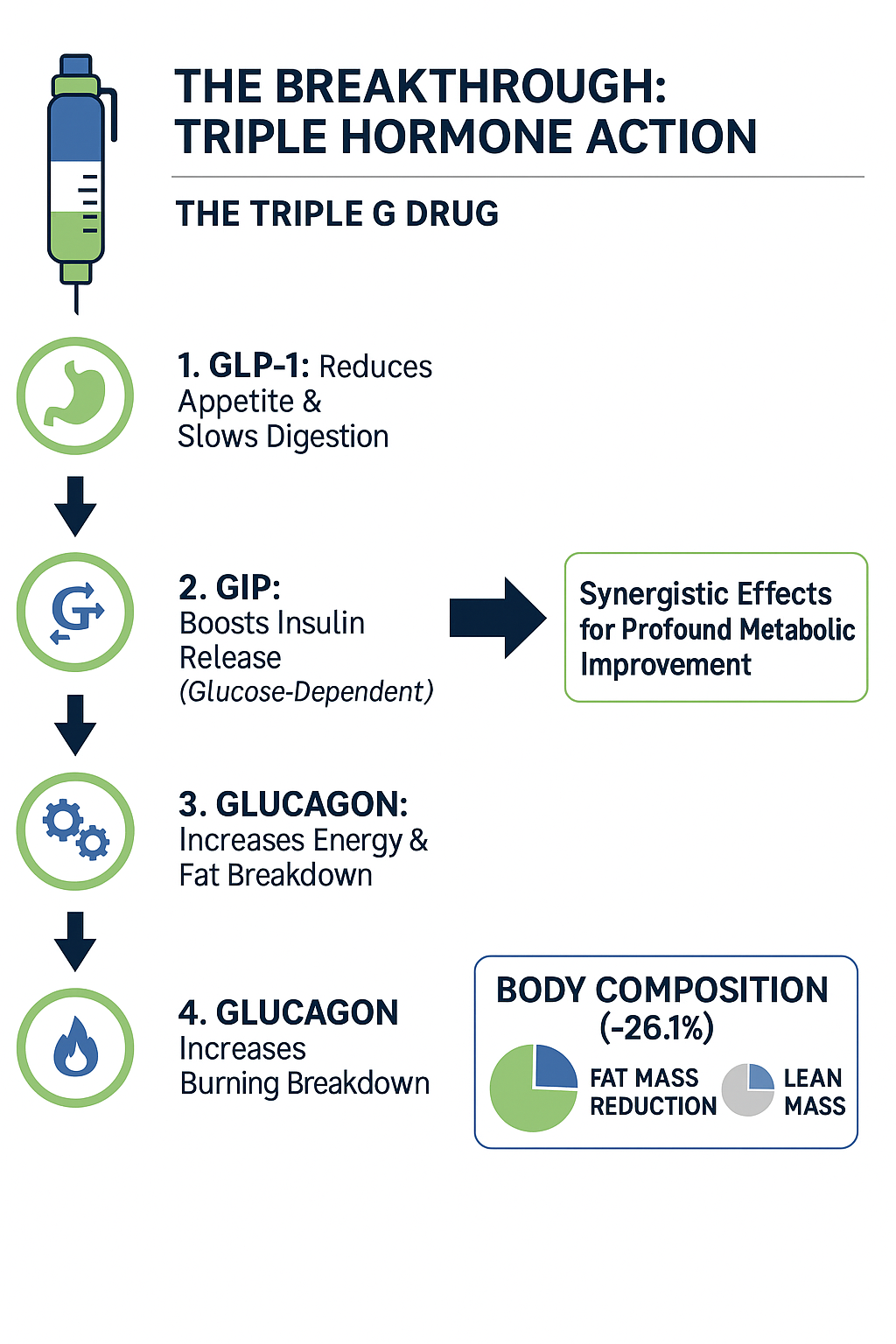
This triple-action mechanism results in a powerful synergy. The GLP-1 and GIP components work together to control blood sugar and suppress appetite, while the glucagon component accelerates fat burning and energy use. For a person with Type 2 diabetes, this combination is revolutionary. It not only addresses high blood sugar (hyperglycaemia) but also tackles one of the primary drivers of the condition: excess body weight, particularly visceral fat. The strong link between weight loss and improved diabetes outcomes is well-established, with bodies like the American Diabetes Association (ADA) highlighting that significant weight reduction can even lead to remission in some cases.
Clinical Breakthroughs in Diabetes Treatment
The theoretical promise of Retatrutide's triple-action mechanism has been powerfully validated by its clinical trial results. The data from the Phase 2 trial, published in The Lancet, has sent waves of excitement through the medical community. The study evaluated the drug's effects on individuals with Type 2 diabetes over 36 weeks, revealing unprecedented efficacy in both blood sugar control and weight loss.
Focus on HbA1c (Glycosylated Haemoglobin) and Glucose Control
A key measure for diabetes management is HbA1c, which reflects average blood glucose levels over the preceding two to three months. According to NICE guidelines in the UK, a target HbA1c for adults with Type 2 diabetes is typically 48 mmol/mol (6.5%) or lower.
In the Phase 2 trial, participants receiving the highest doses of Retatrutide saw their HbA1c levels fall by a staggering average of 2.02% (22.1 mmol/mol). This reduction is significantly greater than that seen with many existing diabetes medications. To put this into perspective, a large number of participants achieved levels that are considered non-diabetic:
- 92% of participants on the 8mg dose achieved an HbA1c of 6.5% or less.
- An incredible 78% of participants on the 12mg dose reached an HbA1c below 5.7%, which is the threshold for normal glucose levels in individuals without diabetes.
These results suggest that Retatrutide has the potential not just to manage Type 2 diabetes, but to normalise blood sugar
The Weight Loss Factor

While glucose control was impressive, the weight-loss results were truly groundbreaking.
Obesity is a major risk factor and complicating factor for Type 2 diabetes, and achieving substantial weight loss is critical for improving insulin sensitivity and overall health.
The trial participants on the highest dose of Retatrutide lost an average of 16.9% of their body weight (around 17.5 kg or 38.6 lbs) in just 36 weeks. A separate Phase 2 trial focusing on obesity, published in The New England Journal of Medicine, showed even more profound results over a longer period, with participants losing up to 24.2% of their body weight at 48 weeks.
This level of weight loss, achieved without intensive surgical intervention, was previously unimaginable for a pharmaceutical drug. Crucially, a substudy using advanced imaging techniques revealed that this weight loss was primarily due to a reduction in fat mass, not muscle.
Participants lost up to 26.1% of their total fat mass while largely preserving lean muscle tissue, which is vital for maintaining metabolic health and physical strength. This targeted fat reduction directly contributes to improved insulin sensitivity and can significantly ease the metabolic burden of Type 2 diabetes.
Retatrutide vs. Mounjaro (Tirzepatide) and Ozempic (Semaglutide)
The Edge: Why Triple Action Matters
The research data strongly suggest that adding glucagon receptor agonism provides a significant clinical advantage. While Mounjaro's dual action demonstrated the benefit of targeting more than one pathway, Retatrutide's third mechanism appears to unlock a new level of efficacy. The glucagon action's ability to increase energy expenditure and burn fat on top of the appetite suppression and insulin regulation from GLP-1 and GIP creates a comprehensive metabolic treatment that tackles the problem from three different angles. This is why experts believe Retatrutide could offer superior and more durable results for both diabetes control and weight reduction.
Crucial Warning: Availability and Safety
While the clinical trial results for Retatrutide are incredibly promising, it is absolutely vital for the public to understand its current status.
Current Investigational Status
Retatrutide is an investigational drug and is NOT approved for public use anywhere in the world yet ( 12 December 2025), including the United Kingdom.
If you see advertisements for Retatrutide or its compounded form on social media like TikTok or Instagram, you should not purchase it, as this substance is currently an investigational drug, has not been approved by the MHRA or other regulatory bodies for prescription or sale, and is only legally available to participants in clinical trials, which are not expected to conclude until 2026 at the earliest; consequently, any website or seller offering it is doing so illegally, and buying unregulated, non-pharmacy grade compounds carries serious health risks, including potential contamination, incorrect dosages, and severe adverse effects.
The Dangers of the Unregulated Trade
The extraordinary public appetite for these groundbreaking new metabolic drugs has, regrettably, given rise to a perilous unregulated trade for unapproved substances. Unscrupulous sellers are frequently peddling these products online, often marketing them dishonestly as "research peptides" in an attempt to sidestep crucial regulations. It is absolutely vital that the public understands the serious risks associated with buying these illicit products:
- Uncertain Dosage and Purity: Products from the unregulated supply chain are subject to no quality control whatsoever. The active substance you receive could be underdosed, overdosed, or, indeed, contain an entirely different active ingredient to what was advertised.
- Absence of Sterility: Injectable medicines must be manufactured in strictly sterile environments. Illicit products are routinely concocted under unsanitary conditions, presenting a significant risk of bacterial contamination and subsequent serious infection.
- Dangerous Contaminants: Vials sourced through these illegal channels may be contaminated with heavy metals, unidentified chemical by-products, or other deeply harmful substances.
- Risk of Severe Side Effects: Taking such a potent, unapproved drug without proper medical supervision could trigger life-threatening adverse reactions, including severe hypoglycaemia (dangerously low blood sugar), pancreatitis, heart complications, and acute allergic reactions.
Regulatory bodies are actively cracking down on this illegal trade. The MHRA recently announced a raid on an illegal manufacturing facility, seizing thousands of doses of unapproved products. This highlights the very real and present danger of the unregulated market.
Conclusion
Retatrutide represents a potential paradigm shift in the treatment of Type 2 diabetes and obesity. Its unique triple-action mechanism has delivered unprecedented results in clinical trials, offering the possibility of not just managing these chronic conditions, but of normalising key metabolic markers to a degree previously thought impossible with medication alone.
The significant reductions in HbA1c and body weight point to a future where medicine moves beyond simple glucose control and towards a more comprehensive and holistic approach to metabolic health. However, excitement must be tempered with patience and caution.
The global medical community now awaits the results of the large-scale Phase 3 trials to confirm these promising findings and, most importantly, to establish a long-term safety profile. Retatrutide is a beacon of hope and a testament to the incredible pace of medical innovation. While it is not a solution for today, it provides a tantalising glimpse into the future of metabolic medicine, a future that looks brighter and more hopeful than ever before.
Don't Wait for Tomorrow
You do not have to wait for Retatrutide to be approved.
You can start transforming your life and health today with currently approved, effective treatments like Wegovy or Mounjaro (where clinically appropriate).
Looking for effective and weight management solutions? Discover our evidence-based weight loss programme at SheMed, designed for lasting results and delivered with clinical excellence.
Key Takeaways from SheMed
- It's a "Triple Threat" Drug: Retatrutide uniquely targets three metabolic hormone receptors (GLP-1, GIP, and glucagon), making it potentially more powerful for blood sugar and weight loss than current single or dual-action drugs.
- Promising But Preliminary: Clinical trial results show unprecedented efficacy (e.g., ~24% weight loss), but it remains an investigational drug. It is NOT approved for use by any regulatory authority worldwide.
- Zero Legal Availability: It will not be available for prescription until at least 2026-2027. Any product being sold now is illegal, counterfeit, and dangerous.
- Severe Safety Warning: Obtaining it outside of clinical trials poses extreme risks, including contamination, incorrect dosing, infection, and severe side effects. Only use MHRA/TGA/FDA-approved medications prescribed by your doctor.
Frequently Asked Questions (FAQs)
- What is Retatrutide?
A "Triple G" investigational drug that targets three hormone receptors for potentially superior blood sugar and weight control. It is not approved anywhere yet. - How does Retatrutide compare to Wegovy or Mounjaro?
It is a triple-hormone agonist (GLP-1, GIP, glucagon), while Wegovy is single and Mounjaro is dual, which may lead to stronger effects. However, it is not yet approved, unlike the others.
References
- Jastreboff, A. M., et al. (2023). Triple–Hormone-Receptor Agonist Retatrutide for Obesity — A Phase 2 Trial. The New England Journal of Medicine, 389(6), 514-526. DOI: 10.1056/NEJMoa2301972.
- Rosenstock, J., et al. (2023). Efficacy and safety of a novel triple hormone receptor agonist retatrutide in people with type 2 diabetes: A randomised, double-blind, placebo and active-controlled, parallel-group, phase 2 trial in the USA. The Lancet. DOI: 10.1016/S0140-6736(23)01727-4.
- American Diabetes Association (2022). Weight Loss is Key to Remission of Type 2 Diabetes. Press Release. Available at: https://diabetes.org/newsroom/press-releases/2022/weight-loss-key-remission-type-2-diabetes.
- National Institute for Health and Care Excellence (NICE). (2022). Type 2 diabetes in adults: management. NG28. Available at: https://www.nice.org.uk/guidance/ng28.
- Medicines and Healthcare products Regulatory Agency (MHRA). (2023). MHRA and partners shut down illegal manufacturing of weight loss products. Press Release. Available at: https://www.gov.uk/government/news/mhra-and-partners-shut-down-illegal-manufacturing-of-weight-loss-products.
Take charge of how you look and feel.
Backed by science. Guided by experts.
SheMed’s medical weight loss programme combines expert care and science-backed treatment to help you feel and look your best — for life.
SheMed’s medical weight loss programme combines expert care and science-backed treatment to help you feel and look your best — for life.
The content on the SheMed blog is provided for general informational and educational purposes only. While SheMed provides professional weight loss services and strives to ensure the information shared is accurate and up to date, we make no representations or guarantees as to its accuracy, completeness, or timeliness. This content should not be taken as personal medical advice or a substitute for consultation with a qualified healthcare provider. Always speak with your doctor or licensed medical professional about your individual health or medical needs before starting any new treatment or programme. Never disregard or delay seeking professional medical advice because of something you have read on this site. SheMed is not responsible for any actions you may take based on the information provided in this blog.
Subscribe to our Newsletter
Thank you! Your submission has been received!
Oops! Something went wrong while submitting the form.


